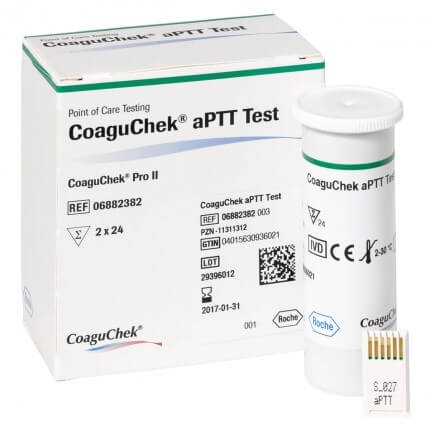
Roche CoaguChek aPTT Test
Test strips for the CoaguChek Pro II system
Item in stock
CoaguChek aPTT Test from Roche
With the CoaguChek aPTT test strips, which are specially designed for the CoaguChek Pro II System from Roche, the activated partial thromoplastin time (aPTT) can be determined quickly and easily within 5 minutes at the point-of-care . This enables mobile monitoring of anticoagulation therapy in real time. The measured values (plasma equivalent seconds) are comparable to the precision of laboratory results. For quality control purposes, a code chip is included in each package, which reads relevant information on the test method, lot number as well as the expiry date into the CoaguChek Pro II system.
Important Note: For healthcare professionals only.
Thetest may only be performed and interpreted by a physician or a person authorized by a physician. Medical laypersons may use and interpret the test incorrectly.
Product details
- Quantitative aPTT determination with the CoaguChek Pro II System
- In vitro test of activated partial thromoplastin time (aPTT)
- Sample application field can be filled from the side or from the top
- Insensitive, heparin-interfering test strips
- Constant monitoring of system and test strips during measurement
- Incl. code chip with information about each new test strip batch
- Detection system: Amperometric (electrochemical) determination after activation of blood coagulation with Celite
- Sample material: capillary blood; venous and arterial whole blood
- Sample volume: 8 μl
- Measuring time: approx. 5 minutes
- Measuring range: 20 - 130 plasma equivalent seconds
- Heparin effects: Insensitive to low molecular weight heparins (LMWH) up to 0.5 IU/mL blood, at UFH: linearity of 0.1-1.0 IU/mL
- Stability: Store at +2 °C to + 30 °C
Delivery contents
- 2 x 24 Roche CoaguChek Pro II aPTT test strips
- 1 Roche CoaguChek Pro II code chip
Note: The test strips can only be used with the Roche CoaguChek Pro II system.
Return policy
This item is not eligible for returns.
For consumers, the right of withdrawal does not apply to contracts for the delivery of sealed goods that are not suitable for return for reasons of health protection or hygiene if their seal has been removed after delivery.
Brand: Roche
Further information

Manufacturer
Roche Diagnostics GmbH
Sandhofer Strasse 116
68305 Mannheim
Germany
mannheim.allgemein@roche.com