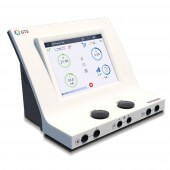
Gymna Duo 400V electrotherapy device
Delivery 35 to 38 days
The delivery will take place between the 14.09.2024 and 18.09.2024Duo 400V electrotherapy device from Gymna
The Duo 400V from Gymna not only uses the technology of the Duo 400, but also comes up trumps with an integrated vacuum unit, which makes it possible for you to perform electrotherapy with vacuum electrodes. The professional therapy device is used for pain relief, tissue repair and muscle stimulation in physiotherapy and rehabilitation. The color touch screen shows a comprehensive program selection with the color guided therapy system (GTS) for easy navigation. Treatment strategies are selectable by treatment goal, indications or body part. Gymna Duo 400V works according to the latest recommendations of evidence-based therapy and enables successful treatment.
Product details
- 2-channel electrotherapy device for pain relief by TENS
- Microcurrent, ultrasound or combination therapy
- Incl. modular vacuum unit with suction electrodes and integrated water tank
- Guided therapy system (GTS) with simple color management
- 2 independent channels, 2- and 4-pole
- 2 separate intensity controllers
- S-D curve diagnostic programs (also I/t curve, Rheobase, Chronaxie, etc)
- With 31 current forms
- Dimensions: W 36 x H 33 x D 28.5 cm
- Weight: approx. 10,8 kg
The functions
Innovative color guidance system
The Guided Therapy System (GTS) stands for intuitive navigation. Electrotherapy, ultrasound therapy or combination therapy, as well as the optional vacuum and laser therapy, have a uniform color design from therapy selection to parameter display. Large selection keys in the display make starting therapy quick and easy. The device settings can also be customized with the setup wizard - for a personal touch and intuitive handling in every application.
- Programs selectable by treatment goal, by body area, indication or cellular effects
- Detailed images for placement of electrodes and devices on the body
- I/T curve diagnostic programs (stimulus intensity / stimulus time curve diagnostics)
- Diagnostic programs on the state of electrical sensitivity of the neuromuscular system (S-D curve)
Electrotherapy
- Duo 400 unit for 2 or 4 rubber electrodes
- Vacuum unit Vaco 400 for suction electrodes
- Two independent channels each, 2-pole controllable
- Simultaneous therapy: 2 independent channels for simultaneous treatment of two different indications
- Currents: rectified, diadynamic, interference, TENS (transcutaneous electrical nerve stimulation), NMES (neuromuscular electrical stimulation), as well as micro and high voltage current
- Vacuum with continuous and pulse mode, massage function
Technical data
- Display: 10.4" TFT LCD touch screen
- Currents: 31, various current forms
- Simultaneous therapy, vacuum
- Vaco 400 vacuum unit: electronic vacuum control
- Functionalities: Treatment target, indications, diagnostics, body part, cellular effects, standard therapy programs, free memory menu, anatomy library
- USB port for software updates
- Languages: 13
- Adjustable speaker for acoustic feedback
- Memory for programs and results (850 locations)
- Medical device: Class IIa
- Safety class II
- Insulation: Type BF
- Mains voltage: 100-240 VAC, 50/60 Hz
- Maximum power, in operation: 100 VA
Scope of delivery
- 1 Gymna Duo 400V electrotherapy unit
- 1 Gymna Vaco 400 Vacuum unit
- 4 rubber electrodes (6 x 8 cm)
- 4 Chamex sponge bags for electrodes
- 4 vacuum electrodes (60 mm)
- 4 round sponges for vacuum electrode (60 mm)
- 4 fixation tapes (5 x 60 cm)
- 2 patient electrode cables, two-core
- 1 connection cable (power supply and communication)
- 1 connection cable (electrotherapy)
- 4 vacuum hoses (2 x light gray, 2 x dark gray)
- 1 Pain scale (Visual analogue scale score card)
- 1 touch pen
- 1 power cord
- 1 Quick start manual
- 1 Instruction CD Gymna Series
The product is subject to instruction in Germany according to MPBetreibV. The instruction is not included in the price.
Return policy
This item is not eligible for returns.
For consumers, the right of withdrawal does not apply to contracts for the delivery of sealed goods that are not suitable for return for reasons of health protection or hygiene if their seal has been removed after delivery.